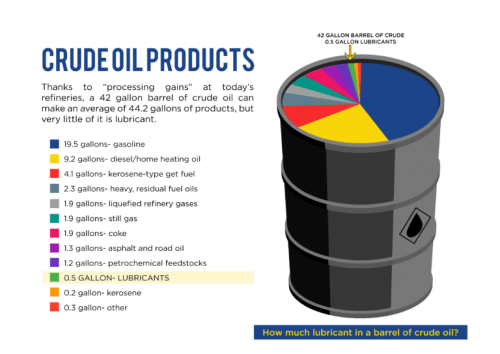
What are Friction Modifiers?
Friction modifiers are mild anti-wear additives used to minimize light surface contact, such as sliding and rolling. These can also be referred to as boundary lubrication additives. These additives are used in lubricants to modify the coefficient of friction (hence the name, Friction Modifiers). Friction modifiers are deployed to prevent wear on metal surfaces. Mostly used in transmission fluids and engine oils, these additives help slow down wear and increase fuel economy.
How do Friction Modifiers Work?
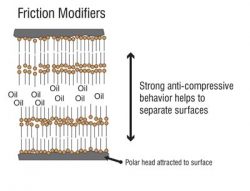
A friction modifier molecule consists of two parts: a polar end (head) and an oil-soluble end (tail). The head attaches itself to the metal surface to create a cushion for the metal surface against another metal surface. The tails stand up like a carpet; vertically stacked besides each other in a Nano-sized sheet covering the metal surface. These molecules hold up when cushioned surfaces come in light contact with each other. This forms a thick boundary film that is softer than metal surfaces.
These additives have multiple functions beyond friction modification. They work as antioxidants and corrosion inhibitors as well. As the contact or load becomes heavier, the polar molecules are brushed off, thus rendering the additive useless in reducing friction.
Friction Modifier Applications
Friction Modifiers are typically used in engine oils and automatic transmission fluids. In engine oils, friction modifiers are deployed to improve fuel economy by reducing friction. In transmission fluids, friction modifiers are deployed to improve engagement on clutches. Some situations require some traction to operate properly.
Their use in engine lubricants increased in the 1970s due to the oil embargo. The lack of fuel led the automotive industry to improve fuel economy, thus reducing fuel usage. Continuous development has led to lower viscosity lubricants. Now, lubricants require robust friction modifiers to reduce wear and friction to offset the lower viscosity.
However, friction modifiers in these applications act differently based on shear conditions. This ensures equipment does not wear while also preventing too much slippage. This smooths the transition from a dynamic condition to static condition. For example, this is used in a gear change in a transmission.
Anti-Wear and Extreme Pressure (EP) Additives
As loads become heavier, engineers must adjust their lubricant to meet the tougher demands of heavier loads and higher temperatures. You should switch to a modifier that is classified as an anti-wear additive. A common and effective anti-wear agent is Zinc dialkyldithiophosphate (ZDDP). These additives react with metal surfaces once the environment reaches a high enough temperature.
As loads continue to increase, in addition to metal contact, the friction modifier must become more robust. In this instance, your lubricant must include extreme pressure (EP) additives. These additives are either temperature-dependent or not. Temperature-dependent EP additives activate as the metal surface temperature increases due to the extreme pressure. The reaction is driven by the heat produced from friction.
Final Thoughts
Lubricants with friction modifiers create more efficient operating environments. This leads to less wear, downtime, and carbon dioxide emissions. As friction modifier additives improve, lubricant manufacturers will aim to reduce to viscosity to reduce shear conditions. Conversely, this creates more components operating in thin boundary lubrication conditions. We will see continuous innovation of these additives to meet the performance and efficiency demands.